Introducing Algeness®

A Breakthrough in All-Natural Dermal Filler Technology
A 100% natural, biodegradable, and moldable solution is now available for correcting wrinkles. Made from a highly purified agarose gel, Algeness® is an injectable implant that provides immediate volumizing to the face, for a younger appearance. Safe and chemical free, Algeness® is fulfilling the expectations of even the most demanding practitioners.
The Problem
As we age, we lose the volume in our face that makes our skin look soft and youthful. Dermal fillers, which can temporarily erase lines and wrinkles, are increasingly popular. However, all dermal fillers are not the same; competing products, such as Hyaluronic Acid (HA), contain chemicals that can cause harmful side effects. This is why both patients and the medical community have been searching for a more natural solution.
Algeness®: The Anti-Aging Solution

Created by Advanced Aesthetics Technologies, Algeness® fulfills patients’ demands for better and safer products to enhance and improve their appearance and sense of well-being. Our product is a 100% natural, biodegradable, and injectable gel implant—the culmination of 10 years of scientific and clinical research. We have received government approvals in the EU and elsewhere, and Algeness® is now being used to treat patients in 27 countries across Europe, Asia, Latin America, and the Middle East.
Algeness®: Competitive Advantages
- 100% biodegradable, fully biocompatible and non-allergenic, offering excellent tolerability for patients, with minimal irritation
- Free of solvents and synthetic chemicals
- Volumizing effects immediately visible, allowing medical practitioners and patients to evaluate the result of the injection immediately after the procedure, compared to the 2-4 weeks required to see the final results of Hyaluronic Acid fillers
- Exhibits low level of migration - the product stays where it has been injected
- Typically requires only one visit, compared to multiple visits required for competing fillers
- Long lasting results of over 12 months, proven by clinical studies
Algeness®: Patented Manufacturing Process
Algeness® is a biomaterial, a highly purified agarose gel, produced from a sophisticated, patented manufacturing process. The gel is derived from the purification and separation of agar-agar, which is processed from red algae.

Algeness® Platform Products
Algeness® is formulated in four injectable concentrations that provide differing levels of volumizing. The Algeness® platform products can create cosmetic improvements of the lips, fine lines, and the folds around the mouth for shaping and volumizing the cheeks, jawline, and other areas.

Master Your Results™ Training Program for Physicians
In order to deliver consistent outcomes for patients, AAT created its Master Your Results™ training program for medical practitioners. All Algeness® practitioners are required to achieve certification in this program. The program covers Algeness® injectable implant technology through live, hands-on workshops. Over 600 plastic surgeons in 27 countries have been trained and certified through Master Your Results; the number of certified plastic surgeons is projected to reach 1000 by the end of the year.

Intellectual Property
AAT holds three issued patents and 11 pending, with claims on composition and methods related to the Algeness® platform products as well as a registered trademark in the Algeness® mark. Several of these patents are subject to exclusive license agreements for our next-generation of products. We have filed 6 patents based on this technology, with claims related to gel implants, absorbable sutures, scaffolding, and active fillers. AAT also has 4 patents pending in the U.S. and internationally with respect to other technology that has been developed by the company.
Market Opportunity
Global Dermal Filler Market is Growing
According to Market Research Future, the global dermal fillers market is expected to grow at a compound annual growth rate of 12.5% over the next five years, and is expected to reach $10 billion by 2023.
Market Size
Worldwide, 7-8 million filler procedures are performed annually by plastic surgeons, dermatologists and other practitioners. In the U.S. alone, plastic surgeons performed nearly 3.5 million Hyaluronic Acid filler procedures in 2016, representing over 25% of the non-surgical procedures performed by these surgeons (2016 International Study of Cosmetic Surgery, International Society of Aesthetic Plastic Surgery).
100% Natural Algeness® is the Next-Generation Technology
Two major brands of Hyaluronic Acid fillers dominate the current dermal filler market. Critically, no competitor has a next-generation formulary that resolves the known complications of Hyaluronic Acid fillers. 100% natural Algeness® is the next-generation technology; competitors may not be able to adequately protect their franchises as consumers seek more natural fillers that produce immediate, stable results.
Other Potential Markets
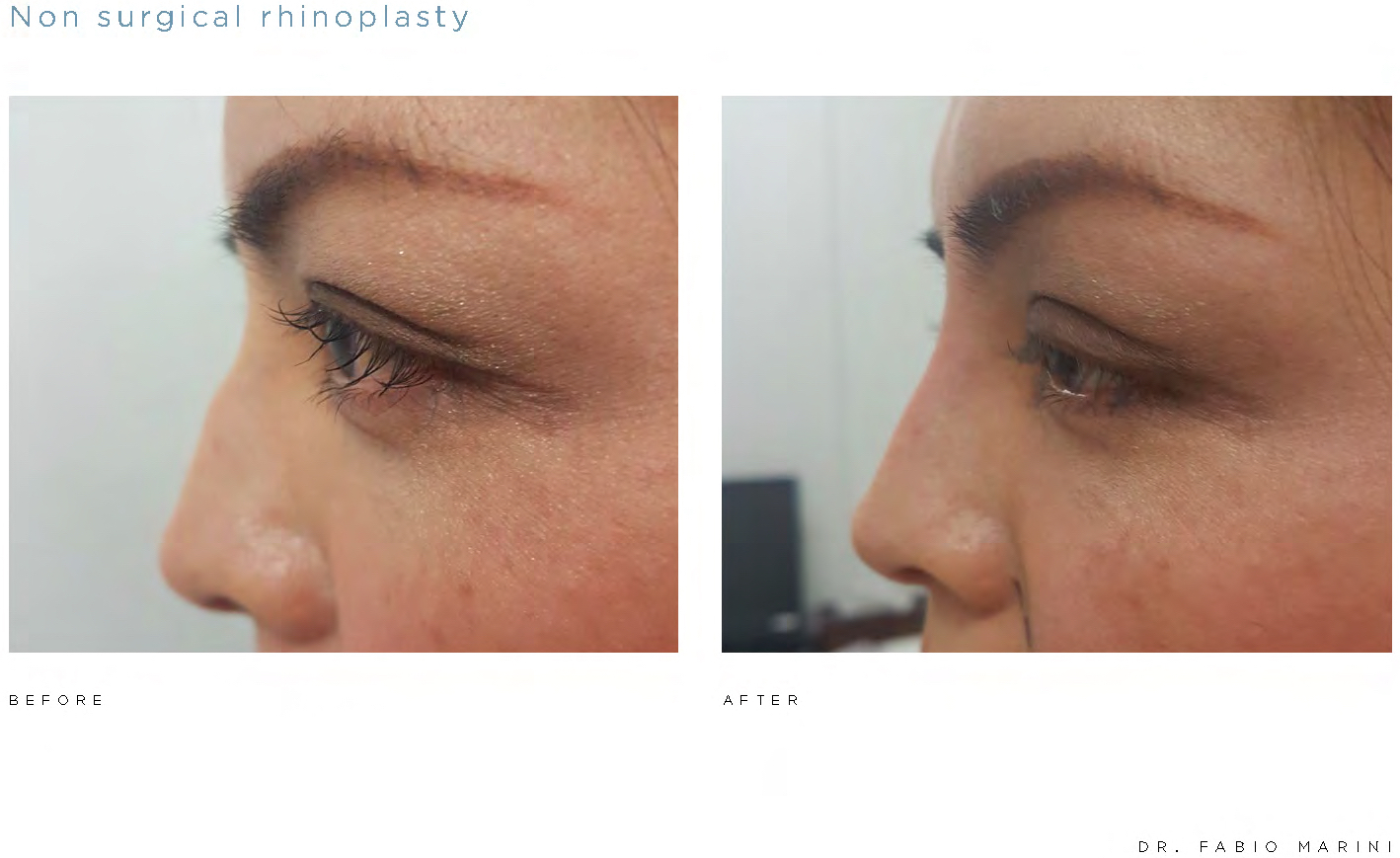
AAT is also exploring the use of the Algeness® injectable gel implant platform in other anatomical areas of the body. In preliminary controlled studies, practitioners have reported encouraging outcomes in the non-surgical therapeutic areas of rhinoplasty and hand volumizing. While research and development with respect to additional medical applications of the Algeness® platform is at an early, exploratory stage, there is promising anecdotal evidence for potential use of Algeness® for body filling, active fillers, wound treatment, and absorbable suture applications.
Business Model
AAT sells pre-filled double syringe packs to the company’s network of authorized country distributors with gross margins in excess of 65%. The Company’s distributors then sell the products to practitioners who have been certified under the Master Your Results program at a markup. Distributors have exclusive sales in their respective countries and undertake the promotion of Algeness®, including organizing Master Your Results training sessions and live workshops and participating in local and regional exhibitions, conferences, and congresses. Distributors earn a margin on products sold to practitioners.

Under an exclusive license and manufacturing agreement, Algeness® is manufactured by Ghimas, an ISO-certified medical device manufacturer in Bologna, Italy. Finished products are shipped to our wholly-owned subsidiary, Algeness-Europe LTD, based in Dublin, Ireland. Algeness-Europe LTD then sells and ships the products to the company’s distributors.
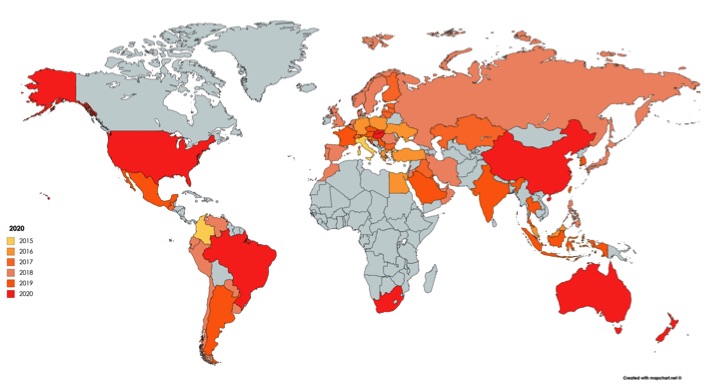
Projected Growth of AAT Distribution Network Through 2020
Success to Date
- AAT generated revenues of more than $300,000 in 2017, up 58% from the previous year.
- AAT has successfully completed Series A and B rounds, raising $5M.
- AAT currently operates in 27 countries in Europe, the Middle East, and Asia, with an additional 16 countries in registration.
- In December 2017, the Company signed a registration and distribution agreement with Nanjing Ouyi Medical Device Ltd (“Nanjing Ouyi”), located in Nanjing, China. Nanjing Ouyi is funding initial studies required to obtain regulatory clearance for the distribution of our products in mainland China. Once regulatory clearance is obtained, Nanjing Ouyi will have exclusive distribution rights in mainland China, subject to certain financial and other performance requirements.
- Algeness® received the CE Mark Certification for the European Economic Area (EEA) signifying that this medical device has met the high safety, health, and environmental protection requirements set by the EEA.
- AAT has started two post-market comparative studies in Europe designed to meet certain industry regulatory standards that are recognized by the FDA.
- Algeness® was recognized for its break-through innovation in dermal fillers at the 10th Anti-Aging Medicine Congress in Bucharest, Romania in 2018.
Recent Events
Team
Mr. Abel brings over 25 years of medical device and specialty pharma experience to AAT, including senior roles at Allergan and Biogen, as well as CEO and Chief Business Officer roles at several start-ups. He was a member of the BOTOX® leadership and launch team that built and executed Allergan's re-entry into dermatology and was the global lead for BOTOX® aesthetic and hyperhidrosis development and commercial launch efforts. As VP of Dermatology at Biogen, he built and led the team that launched the first Biologic approved for psoriasis. Also, while General Manager of Onset Dermatologics, he led the development of multiple products and grew revenue by more than 6-fold. He holds a BA in Chemistry from Lafayette College and an MBA from Temple University.
Mr. Burtt brings over 35 years of senior management experience to Advanced Aesthetic Technologies, Inc., including previous positions at Medtronic and IBM. He has raised over $50 million in angel and venture capital in his management capacity and co-founded or led five companies to acquisitions. Mr. Burtt holds an MS in Chemical Engineering from the University of Massachusetts and the Professional Director Certification for Public Companies from the American College of Corporate Directors.
A board-certified plastic surgeon, Dr. Miller has more than 25 years of experience in cosmetic plastic surgery and facial rejuvenation procedures. He helped found PureTech Ventures, a biomedical seed fund, and Environ USA, a cosmeceutical company. As the Clinical Founder of ThermiAesthetics, Inc., he helped develop a therma-controlled radiofrequency technology for skin tightening and nerve ablation. He is currently the CEO of Medical Aesthetic Technology Corporation (MATC), which develops nanotechnology for cosmetic delivery systems. Dr. Miller attended medical school at the University of Cape Town and was further trained at Groote Schuur Hospital, Peter Bent Brigham Hospital, Harvard Surgical Services, and Emory University Affiliated Hospitals. In addition to running a leading private plastic surgery practice in Boston, Dr. Miller serves as a Clinical Instructor in Surgery at Harvard Medical School and as a staff surgeon at Beth Israel Deaconess Hospital.
John Preston is the former Director of Technology Development (and Licensing) at MIT where he was responsible for the commercialization of MIT-developed technologies. Of the companies started during his tenure, those that went public have a cumulative market capitalization estimated to be above $100 billion. Mr. Preston was awarded the rank of “Knight of the Order of National Merit of France” by French President Mitterrand and the “Hammer Award for Reinventing Government” by Vice President Gore. He chaired President George H. W. Bush’s conference announcing the President’s technology initiative and has previously served as a Board Advisor to Mars Incorporated.
A board-certified plastic surgeon, Dr. Kinney is Deputy Secretary General of the International Confederation of Plastic, Reconstructive and Aesthetic Plastic Surgeons (IPRAS), former member of the Board of Directors of the American Society of Plastic Surgeons, past President of the Plastic Surgery Educational Foundation and past Chairman of the Board of Trustees of the American Society of Plastic Surgeons. A staff member of St. John’s Hospital and Health Center and Cedars-Sinai Medical Center, Dr. Kinney is also a Clinical Associate Professor of Plastic Surgery at USC School of Medicine. He serves as a consultant to biotech and engineering companies and has authored numerous journal and magazine articles and co-edited a standard textbook of Plastic Surgery, Plastic Surgery: Indications and Practice. Dr. Kinney earned his bachelor’s and master’s degree in the Harvard/MIT Health Sciences and Technology program. He received his Medical degree at Tulane University Medical School and trained in general surgery and plastic surgery at UCLA Medical Center. As a team leader in new technologies, Dr. Kinney has participated in three US FDA clearances and in numerous FDA clinical trials.
Mr. Sayare spent over 25 years as CEO and Founder of ImmunoGen, Inc. During his tenure, he led the Company through four private rounds of venture financing and to an IPO in 1989, totaling over $500 million in capital formation. Dr. Sayare also serves as Chairman of the Board of PharmaAthene, Inc., is a Director of Boston IVF, Isabella Products, and Cymogen DX. He is a member of the scientific advisory board of LA BioMed and the Harbor-UCLA Medical Center, where he also serves as a member of the board executive committee. Dr. Sayare holds a PhD in biochemistry from Temple University Medical School and was a former assistant professor of biochemistry at the University of Connecticut.
Mr. Rogal’s career spans over 30 years in the high tech electronics industry. He is currently Chairman and CEO of Gold Circuit Electronics, a leading manufacturer of high technology printed circuit boards. Prior to Gold Circuit, Peter founded Comtel Electronics and Comtel Security Systems, which were acquired by Palomar Corporation in 1998. He was also Chairman and CEO of Beacon Endoscopic, Inc., which was recently acquired by Covidien, and was founder of Brooktrout Ventures, an investment and marketing firm. Mr. Rogal is a graduate of Ithaca College.
For the last 9 years, Paul has been the owner and managing director of Elzer Services LTD, which provides accounting and financial assistance to hedge funds, insurance companies, and other clients. He also serves as a director, and managing director of Algeness - Europe, AAT’s European subsidiary. Mr. Sinnott has served as an independent non-executive director, Finance Director and Chairman on a number of various Boards. He has worked for over thirty five years in a number of financial roles across a wide variety of industry sectors ranging from financial services to software development to the leisure industry. Paul has a hands on, common sense, can do approach with an open collaborative style. He is results orientated with a proven track record of developing businesses to their true potential. He is an experienced private investor and has been a member of the Institute of Chartered Accountants in Ireland since 1981. He has served as a director of AAT since 1/09/2018.
John Cammett is Co‐Founder of Realterm Global, a vertically integrated industrial real estate development and investment management company, with a primary focus on transportation facilities serving the global supply chain. Realterm owns and manages over 300 facilities totaling $4.5 billion in investment. John led the company from its initial air cargo development in Montreal, Quebec in 1991 to its current airport industrial real estate portfolio of 35 airports. He is a graduate of Harvard College, with a bachelor’s degree in engineering and applied sciences.
Mr. Gitto has spent 20 years as an Executive Director in the medical and beauty industries. Prior to his position as AAT’s COO, he was COO for LPG Systems, world leader in the production of medical devices for cellulite treatment, body contouring, and physiotherapy indications. He was also Vice President of Siyanli Co. Ltd, one of the largest high end spa and medical-spa and aesthetic clinics in China. He was also a board member of the Sophia Biotech association in France. He is fluent in 5 languages, is graduated in economics and management and has an advanced degree in marketing from the Universite Nice Sophia Antipolis, in France.
Mr Tinkham has had a successful career in both early stage and large cap healthcare companies, both operationally as well as M&A experiences. He is currently President & CEO of GI Windows a company in which he was an original founder. He is recognized as a leader in innovation and entrepreneurship as an invited speaker and faculty for multiple Gastroenterology & Hepatology societies both US and International. His past experiences include, Vice President of Sales and Vice President of New Technologies for Medtronic. Prior to Medtronic, Mr Tinkham was the Co-Founder of Beacon Endoscopic (acquired by Covidien 2014) and held global marketing & sales leadership positions at Boston Scientific.
Use of Proceeds
Proceeds of the offering will be used to:
- Help finance clinical and other studies in connection with obtaining Investigational Device Exemption from the U.S. Food and Drug Administration (“FDA”) and, ultimately, market clearance in the U.S;
- Advance product development for non-surgical therapeutics and new gel implant applications; and
- Scale the company’s sales and marketing efforts.
If the offering's maximum Reg CF allocation of $1,070,000 is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| Working Capital | $1,017,570 | 95.1% |
| Intermediary fees | $52,430 | 4.9% |
If the offering's maximum amount of $4,000,000 across Reg. CF and Reg. D is raised:
| Use | Value | % of Proceeds |
|---|---|---|
| FDA Clinical Study | $3,000,000 | 75.0% |
| Product Development | $402,000 | 10.1% |
| Infrastructure Scale Up | $402,000 | 10.1% |
| Intermediary fees | $196,000 | 4.9% |
Terms
This is a side-by-side offering of Common Stock, under registration exemptions 4(a)(6) and 506(c), in Zava, doing business as Photojam. Up to $1,070,000 may be raised under the 4(a)(6) exemption. Netcapital will determine which exemption applies to your investment and notify you before you complete your investment.
The amount raised under the two exemptions must total at least $10,000 by March 15, 2019 at 6:59pm ET. If the total doesn’t reach its target, then your money will be refunded. Photojam may issue additional securities to raise up to $4,000,000, the offering’s maximum.
If the side-by-side offering is successful at raising the maximum amount, then the company’s implied valuation after the offering (sometimes called its post-money valuation) will be:
Financials
Photojam’s official name is Zava, so that’s the name that appears in the statements below.
These financial statements have been audited by an independent Certified Public Accountant.
SEC Filings
The Offering Statement is a formal description of the company and this transaction. It’s filed with the SEC to comply with the requirements of exemptions 4(a)(6) and 506(c) of the Securities Act of 1933. Similar information is sometimes offered in a Private Placement Memorandum for 506(c) offerings.
We’re also required to share links to each of the SEC filings related to this side-by-side offering with investors.
Understand the Risks
Be sure to understand the risks of this type of investment. No regulatory body (not the SEC, not any state regulator) has passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials or information posted herein. That’s typical for Regulation CF or Regulation D offerings like this one.
Neither Netcapital nor any of its directors, officers, employees, representatives, affiliates, or agents shall have any liability whatsoever arising from any error or incompleteness of fact or opinion in, or lack of care in the preparation or publication of, the materials and communication herein or the terms or valuation of any securities offering.
The information contained herein includes forward-looking statements. These statements relate to future events or to future financial performance, and involve known and unknown risks, uncertainties, and other factors, that may cause actual results to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond the company’s control and which could, and likely will, materially affect actual results, levels of activity, performance, or achievements. Any forward-looking statement reflects the current views with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to operations, results of operations, growth strategy, and liquidity. No obligation exists to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
More Info
Updates
- Mar 15, 2019Primary offering finalized, selling shares
Ask a Question
Proofread your comment before submitting: once it's posted, you can’t edit or delete it. Investors are advised to review our Discussion Board Policy before submitting a comment. For the fastest help with the web site, email help@netcapital.com instead of commenting.
Looking to raise capital?
We can help turn your friends, family and customers into investors.
Interested in more investment opportunities?
Browse all offerings currently available.
